A Four-Session Sleep Intervention Program Improves Sleep for Older Adult Day Health Care Participants: Results of a Randomized Controlled Trial
- PMID: 28482053
- PMCID: PMC5804980
- DOI: 10.1093/sleep/zsx079
A Four-Session Sleep Intervention Program Improves Sleep for Older Adult Day Health Care Participants: Results of a Randomized Controlled Trial
Abstract
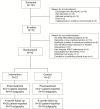
Study objective: To test the effectiveness of a 4-week behavioral Sleep Intervention Program (SIP: sleep compression, modified stimulus control, and sleep hygiene) compared to a 4-week information-only control (IC) among older adults attending a VA Adult Day Health Care (ADHC) program in a double-blind, randomized, clinical trial.
Methods: Forty-two individuals (mean age: 77 years, 93% male) enrolled in a VA ADHC program were randomized to receive SIP or IC. All completed in-person sleep and health assessments at baseline, post-treatment and 4-months follow-up that included 3 days/nights of wrist actigraphy, the Pittsburgh Sleep Quality Index (PSQI), and the Insomnia Severity Index (ISI). Mixed repeated measures analysis was used to compare sleep outcomes at post-treatment and 4-months follow-up, with baseline values as covariates.
Results: SIP participants (n = 21) showed significant improvement on actigraphy sleep efficiency (p = .007), number of nighttime awakenings (p = .016), and minutes awake at night (p = .001) at post-treatment, compared to IC participants (n = 21). Benefits were slightly attenuated but remained significant at 4-month follow-up (all p's < .05). There were no differences in total sleep time between groups. There was significant improvement on PSQI factor 3 (daily disturbances) at 4-month follow-up (p = .016), but no differences were observed between SIP and IC on other PSQI components or ISI scores at post-treatment or 4-month follow-up.
Conclusions: A short behavioral sleep intervention may have important benefits in improving objectively measured sleep in older adults participating in ADHC. Future studies are needed to study implementation of this intervention into routine clinical care within ADHC.
Trial registration: ClinicalTrials.gov NCT01259401.
Keywords: adult day health care; aging; behavioral interventions; sleep; veterans.
Published by Oxford University Press on behalf of Sleep Research Society (SRS) 2017. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

Similar articles
-
Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial.J Am Geriatr Soc. 2016 Sep;64(9):1830-8. doi: 10.1111/jgs.14304. Epub 2016 Aug 22. J Am Geriatr Soc. 2016. PMID: 27550552 Free PMC article. Clinical Trial.
-
Efficacy of Cognitive Behavioral Therapy for Insomnia in Adolescents: A Randomized Controlled Trial with Internet Therapy, Group Therapy and A Waiting List Condition.Sleep. 2015 Dec 1;38(12):1913-26. doi: 10.5665/sleep.5240. Sleep. 2015. PMID: 26158889 Free PMC article. Clinical Trial.
-
Effects of Tai Chi or Exercise on Sleep in Older Adults With Insomnia: A Randomized Clinical Trial.JAMA Netw Open. 2021 Feb 1;4(2):e2037199. doi: 10.1001/jamanetworkopen.2020.37199. JAMA Netw Open. 2021. PMID: 33587135 Free PMC article. Clinical Trial.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.Health Technol Assess. 2004 Feb;8(8):iii-iv, 1-68. doi: 10.3310/hta8080. Health Technol Assess. 2004. PMID: 14960254 Review.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
Cited by 14 articles
-
Effect of Sleep Intervention Programs during Cardiac Rehabilitation on the Sleep Quality of Heart Patients.Sleep Disord. 2022 Mar 24;2022:8269799. doi: 10.1155/2022/8269799. eCollection 2022. Sleep Disord. 2022. PMID: 35368746 Free PMC article.
-
Development and palliative care staff reactions to a sleep regulation educational intervention.BMC Palliat Care. 2022 Jan 21;21(1):12. doi: 10.1186/s12904-022-00902-x. BMC Palliat Care. 2022. PMID: 35062933 Free PMC article.
-
The Impact of Cognitive Behavioral Therapy for Insomnia on Sleep Log and Actigraphy Outcomes in People with Multiple Sclerosis: A Secondary Analysis.Nat Sci Sleep. 2021 Oct 12;13:1865-1874. doi: 10.2147/NSS.S324879. eCollection 2021. Nat Sci Sleep. 2021. PMID: 34675730 Free PMC article.
-
Posttraumatic Stress Disorder Risk and Benzodiazepine Dependence in Older Veterans with Insomnia Symptoms.Clin Gerontol. 2022 Mar-Apr;45(2):414-418. doi: 10.1080/07317115.2021.1954123. Epub 2021 Aug 4. Clin Gerontol. 2022. PMID: 34346855
-
Sleep-Based Interventions in Alzheimer's Disease: Promising Approaches from Prevention to Treatment along the Disease Trajectory.Pharmaceuticals (Basel). 2021 Apr 19;14(4):383. doi: 10.3390/ph14040383. Pharmaceuticals (Basel). 2021. PMID: 33921870 Free PMC article. Review.
Publication types
MeSH terms
Associated data
Grant support
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

