Mindfulness-Oriented Recovery Enhancement vs Supportive Group Therapy for Co-occurring Opioid Misuse and Chronic Pain in Primary Care: A Randomized Clinical Trial
- PMID: 35226053
- PMCID: PMC8886485
- DOI: 10.1001/jamainternmed.2022.0033
Mindfulness-Oriented Recovery Enhancement vs Supportive Group Therapy for Co-occurring Opioid Misuse and Chronic Pain in Primary Care: A Randomized Clinical Trial
Abstract
Importance: Successful treatment of opioid misuse among people with chronic pain has proven elusive. Guidelines recommend nonopioid therapies, but the efficacy of mindfulness-based interventions for opioid misuse is uncertain.
Objective: To evaluate the efficacy of Mindfulness-Oriented Recovery Enhancement (MORE) for the reduction of opioid misuse and chronic pain.
Design, setting, and participants: This interviewer-blinded randomized clinical trial enrolled patients from primary care clinics in Utah between January 4, 2016, and January 16, 2020. The study included 250 adults with chronic pain receiving long-term opioid therapy who were misusing opioid medications.
Interventions: Treatment with MORE (comprising training in mindfulness, reappraisal, and savoring positive experiences) or supportive group psychotherapy (control condition) across 8 weekly 2-hour group sessions.
Main outcomes and measures: Primary outcomes were (1) opioid misuse assessed by the Drug Misuse Index (self-report, interview, and urine screen) and (2) pain severity and pain-related functional interference, assessed by subscale scores on the Brief Pain Inventory through 9 months of follow-up. Secondary outcomes were opioid dose, emotional distress, and ecological momentary assessments of opioid craving. The minimum intervention dose was defined as 4 or more completed sessions of MORE or supportive group psychotherapy.
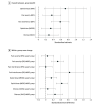
Results: Among 250 participants (159 women [63.6%]; mean [SD] age, 51.8 [11.9] years), 129 were randomized to the MORE group and 121 to the supportive psychotherapy group. Overall, 17 participants (6.8%) were Hispanic or Latino, 218 (87.2%) were White, and 15 (6.0%) were of other races and/or ethnicities (2 American Indian, 3 Asian, 1 Black, 2 Pacific Islander, and 7 did not specify). At baseline, the mean duration of pain was 14.7 years (range, 1-60 years), and the mean (SD) morphine-equivalent opioid dose was 101.0 (266.3) mg (IQR, 16.0-90.0 mg). A total of 203 participants (81.2%) received the minimum intervention dose (mean [SD], 5.7 [2.2] sessions); at 9 months, 92 of 250 participants (36.8%) discontinued the study. The overall odds ratio for reduction in opioid misuse through the 9-month follow-up period in the MORE group compared with the supportive psychotherapy group was 2.06 (95% CI, 1.17-3.61; P = .01). At 9 months, 36 of 80 participants (45.0%) in the MORE group were no longer misusing opioids compared with 19 of 78 participants (24.4%) in the supportive psychotherapy group. Mixed models demonstrated that MORE was superior to supportive psychotherapy through 9 months of follow-up for pain severity (between-group effect: 0.49; 95% CI, 0.17-0.81; P = .003) and pain-related functional interference (between-group effect: 1.07; 95% CI, 0.64-1.50; P < .001). Participants in the MORE group reduced their opioid dose to a greater extent than those in the supportive psychotherapy group. The MORE group also had lower emotional distress and opioid craving.
Conclusions and relevance: In this randomized clinical trial, among adult participants in a primary care setting, the MORE intervention led to sustained improvements in opioid misuse and chronic pain symptoms and reductions in opioid dosing, emotional distress, and opioid craving compared with supportive group psychotherapy. Despite attrition caused by the COVID-19 pandemic and the vulnerability of the sample, MORE appeared to be efficacious for reducing opioid misuse among adults with chronic pain.
Trial registration: ClinicalTrials.gov Identifier: NCT02602535.
Conflict of interest statement
Figures

Cited by 1 article
-
Heightened autonomic reactivity to negative affective stimuli among active duty soldiers with PTSD and opioid-treated chronic pain.Psychiatry Res. 2022 Mar;309:114394. doi: 10.1016/j.psychres.2022.114394. Epub 2022 Jan 14. Psychiatry Res. 2022. PMID: 35066311
References
-
- Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10(2):131-146. doi:10.1016/j.jpain.2008.10.009 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

