Targeting the Kynurenine Pathway for the Treatment of Cisplatin-Resistant Lung Cancer
- PMID: 31628200
- PMCID: PMC7262740
- DOI: 10.1158/1541-7786.MCR-19-0239
Targeting the Kynurenine Pathway for the Treatment of Cisplatin-Resistant Lung Cancer
Abstract
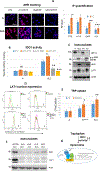
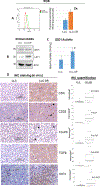
Cisplatin resistance is a major barrier in the effective treatment of lung cancer. Cisplatin-resistant (CR) lung cancer cells do not primarily use glucose but rather consume amino acids such as glutamine and tryptophan (Trp) for survival. CR cells activate the kynurenine (KYN) pathway (KP) to cope with excessive reactive oxygen species (ROS) and maintain homeostasis for growth and proliferation. Consequently, indoleamine 2,3-dioxygenase-1 (IDO1) becomes an essential enzyme for CR cells' survival because it initiates and regulates the first step in the KP. Increased IDO1 activities and ROS levels are found in CR cells versus cisplatin-sensitive lung cancer. Importantly, significantly greater KYN/Trp ratio (P = 0.005) is detected in serum of patients who fail cisplatin when compared with naïve treatment. Knocking down IDO1 using shRNA or IDO1 inhibitors heightens ROS levels and results in a significant growth inhibitory effect only on CR cells and not on cisplatin-sensitive cells. Exposing CR cells to antioxidant (TIRON) results in suppression of IDO1 activity and confers resistance to IDO1 inhibition, indicating an interrelationship between ROS and IDO1. Because KYN plays a critical role in reprogramming naïve T cells to the immune-suppressive regulatory T-cell (T-reg) phenotype, we observed higher expression of TGFβ, FoxP3, and CD4+CD25+ in mice bearing CR tumors compared with tumors from cisplatin-sensitive counterparts. IMPLICATIONS: Findings suggest that the enzyme-inhibitory activity and antitumor efficacy of IDO1 inhibitors rely in part on ROS levels, arguing that IDO1 expression alone may be insufficient to determine the clinical benefits for this class of experimental cancer drugs. Importantly, IDO1 inhibitors may be more suitable to treat patients with lung cancer who failed cisplatin therapy than naïve treatment patients.
©2019 American Association for Cancer Research.
Conflict of interest statement
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Figures




Similar articles
-
Galectin 3 protects from cisplatin-induced acute kidney injury by promoting TLR-2-dependent activation of IDO1/Kynurenine pathway in renal DCs.Theranostics. 2019 Aug 14;9(20):5976-6001. doi: 10.7150/thno.33959. eCollection 2019. Theranostics. 2019. PMID: 31534532 Free PMC article.
-
Kynurenine/Tryptophan Ratio as a Potential Blood-Based Biomarker in Non-Small Cell Lung Cancer.Int J Mol Sci. 2021 Apr 22;22(9):4403. doi: 10.3390/ijms22094403. Int J Mol Sci. 2021. PMID: 33922388 Free PMC article.
-
Design, Synthesis and Biological Evaluation of Novel 1,2,5-Oxadiazol-3- Carboximidamide Derivatives as Indoleamine 2, 3-Dioxygenase 1 (IDO1) Inhibitors.Anticancer Agents Med Chem. 2020;20(13):1592-1603. doi: 10.2174/1871520620666200604121225. Anticancer Agents Med Chem. 2020. PMID: 32496990
-
Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis.Clin Cancer Res. 2019 Mar 1;25(5):1462-1471. doi: 10.1158/1078-0432.CCR-18-2882. Epub 2018 Oct 30. Clin Cancer Res. 2019. PMID: 30377198 Free PMC article. Review.
-
Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities.Trends Pharmacol Sci. 2018 Mar;39(3):307-325. doi: 10.1016/j.tips.2017.11.007. Epub 2017 Dec 15. Trends Pharmacol Sci. 2018. PMID: 29254698 Review.
Cited by 13 articles
-
Superior antitumor immunotherapy efficacy of kynureninase modified CAR-T cells through targeting kynurenine metabolism.Oncoimmunology. 2022 Mar 25;11(1):2055703. doi: 10.1080/2162402X.2022.2055703. eCollection 2022. Oncoimmunology. 2022. PMID: 35355679 Free PMC article.
-
Tryptophan and Its Metabolites in Lung Cancer: Basic Functions and Clinical Significance.Front Oncol. 2021 Aug 6;11:707277. doi: 10.3389/fonc.2021.707277. eCollection 2021. Front Oncol. 2021. PMID: 34422661 Free PMC article. Review.
-
Cisplatin Resistance and Redox-Metabolic Vulnerability: A Second Alteration.Int J Mol Sci. 2021 Jul 9;22(14):7379. doi: 10.3390/ijms22147379. Int J Mol Sci. 2021. PMID: 34298999 Free PMC article. Review.
-
The Role of Tumour Metabolism in Cisplatin Resistance.Front Mol Biosci. 2021 Jun 23;8:691795. doi: 10.3389/fmolb.2021.691795. eCollection 2021. Front Mol Biosci. 2021. PMID: 34250022 Free PMC article. Review.
-
Kynurenines as a Novel Target for the Treatment of Malignancies.Pharmaceuticals (Basel). 2021 Jun 23;14(7):606. doi: 10.3390/ph14070606. Pharmaceuticals (Basel). 2021. PMID: 34201791 Free PMC article. Review.
Publication types
MeSH terms
Substances
Grant support
LinkOut - more resources
Full Text Sources
Medical
Research Materials

